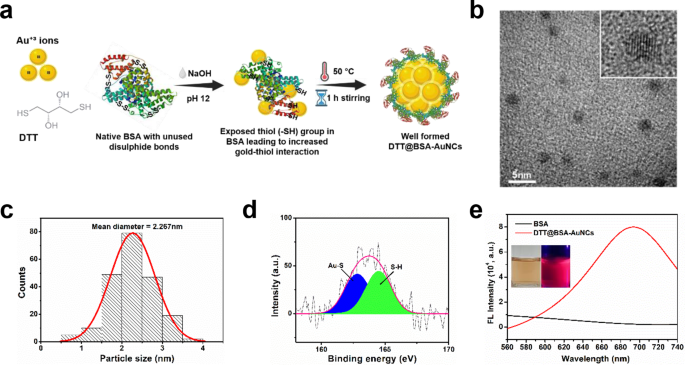
Characterization of DTT@BSA-AuNCs
A schematic illustration of the synthesis course of for DTT@BSA-AuNCs is offered in Fig. 2a. An in depth clarification of every step within the DTT@BSA-AuNCs synthesis, together with the optimization of situations reminiscent of pH and temperature, is offered within the supplementary part (Determine S1–S4 in ESM). The high-resolution transmission electron microscopy (HRTEM) picture of the ready DTT@BSA-AuNCs signifies monodispersed nanoclusters with a mean diameter of two.267 nm (Fig. 2b and c), which falls inside the anticipated dimension vary of Au25NCs, according to earlier studies [41, 42]. Furthermore, the HRTEM photographs confirmed the presence of a regular face-centered unit cell (FCC) construction of Au. As displayed in Fig. 2d, the S 2p XPS peak at 162.8 eV corresponds to the sulfur atom certain to the gold floor as thiolate species [43] and the height at 164.4 eV corresponds to the unbound or free thiol group. Further characterizations of DTT@BSA-AuNCs, as revealed by X-ray diffraction (XRD) evaluation, Au 4f spectrum and survey scan of X-ray photoelectron spectroscopy (XPS), Fourier Remodel Infrared (FTIR) spectrum evaluation are detailed within the supplementary part, together with Figures S5–S8 in ESM.
Synthesis and Characterization of DTT@BSA-AuNCs. (a) Schematic illustration of the synthesis of DTT@BSA-AuNCs. Au(III) and DTT have been launched into BSA, adopted by NaOH addition till the pH reached 12. The uncovered thiol teams in BSA led to elevated gold-thiol interplay. The temperature of the answer was raised to 50 °C with steady stirring for 1 h, resulting in the profitable formation of fluorescent DTT@BSA-AuNCs. (b) HRTEM picture of monodispersed DTT@BSA-AuNCs. The inset within the higher proper nook exhibits an enlarged image of a single AuNC. The size bar is 5 nm. (c) The corresponding dimension distribution of the DTT@BSA-AuNCs exhibits a mean diameter of two.267 nm. (d) XPS evaluation of the sulfur S 2p peak. The S 2p peak at 162.8 eV corresponds to the Au-S bond, and the height at 164.4 eV corresponds to the free thiol group (S-H). (e) Fluorescence emission spectra of DTT@BSA-AuNCs. The DTT@BSA-AuNCs (purple curve) exhibit most fluorescence depth at 690 nm, whereas BSA alone (black curve) has negligible fluorescence at that wavelength. Inset: images of the DTT@BSA-AuNCs below daylight (left) exhibiting an orange-brown coloration, and below 365 nm UV mild (proper) exhibiting brilliant purple fluorescence
As proven in Fig. 2e, the DTT@BSA-AuNCs exhibited most purple fluorescence emission at roughly 690 nm, according to earlier research involving BSA-AuNCs [44, 45] when excited at 510 nm. In distinction, negligible fluorescence emission was noticed from BSA alone. Thus, the sturdy purple fluorescence exhibited by DTT@BSA-AuNCs was attributed to the AuNCs quite than the fluorescence from the BSA protein. The obtained fluorescence emission peak close to 700 nm signifies the presence of Au25 clusters primarily based on the spherical Jellium mannequin. The UV–vis absorption spectrum shows an absorbance band between 280 and 290 nm for DTT@BSA-AuNCs and a distinguished absorbance peak at 280 nm for less than BSA (Determine S3 within the ESM). This phenomenon attributed to the π–π* transitions in fragrant amino acid residues reminiscent of tryptophan, tyrosine, and phenylalanine within the native BSA construction [46]. The slight redshift within the absorbance of DTT@BSA-AuNCs is attributed to the interplay between the AuNCs and the fragrant amino acids in BSA [47]. The absence of a floor plasmon resonance peak at 520 nm, attribute of AuNPs bigger than 3 nm, confirms that no bigger nanoparticles have been fashioned throughout synthesis, according to earlier studies [48].
Picture-responsive oxidase mimicking exercise of DTT@BSA-AuNCs
In any detection course of using AuNCs as nanozymes, a substrate undergoes a catalytic response, resulting in the manufacturing of an electrochemical, fluorescent, or colorimetric response [49]. Notably, the prevalent alternative for readout together with AuNCs has been colorimetric. Nevertheless, because of the superior sensitivity and selectivity of fluorescence over colorimetric detection, this research opted for the fluorescence methodology. TH is a well-liked substrate for fluorescence evaluation because of its cost-effectiveness, excessive water solubility, and huge availability [50]. Initially, being non-fluorescent, TH may be oxidized right into a fluorescent substance known as thiochrome within the presence of applicable oxidants in an alkaline surroundings. The necessity for such alkaline situations limits its utility in biomedical purposes. Nevertheless, in our research, we noticed the oxidation of TH to TC below impartial situations within the presence of sunshine.
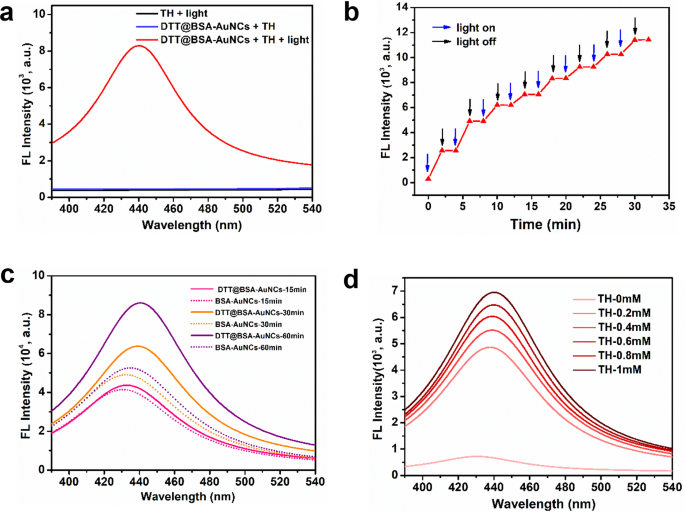
An LED mild with a wavelength of 420 nm was utilized to irradiate the DTT@BSA-AuNCs. Determine 3a, clearly exhibits that when subjected to mild irradiation, DTT@BSA-AuNCs catalyzed the conversion of non-fluorescent TH to fluorescent TC, as indicated by the sturdy fluorescence peak at roughly 440 nm. Intriguingly, within the absence of sunshine publicity, there was a negligible fluorescence sign when TH was handled with DTT@BSA-AuNCs. Moreover, even below mild irradiation, TH alone fails to generate fluorescent TC within the absence of DTT@BSA-AuNCs. These findings point out the indispensable function of seen mild within the catalytic exercise of DTT@BSA-AuNCs nanozyme. Moreover, this end result overcomes the frequent limitation of requiring alkaline situations for TH oxidation, demonstrating that TH may be effectively oxidized to TC below impartial situations. As proven in Fig. 3b, by successively turning on and off the supply of sunshine, the oxidase-mimicking exercise of the DTT@BSA-AuNCs revealed a staircase-like conduct, indicating a photoregulated oxidase-like exercise of the DTT@BSA-AuNCs. Therefore, by way of exterior mild irradiation, the oxidase-like exercise of the DTT@BSA-AuNCs might be exactly managed, favoring excessive spatial-temporal decision of its catalytic exercise. The gradual enhance within the oxidase-mimicking exercise of DTT@BSA-AuNCs with repeated mild irradiation cycles may be attributed to a cumulative impact. Every “mild on” interval results in TH oxidation and the era of TC resulting in TC accumulation over time; therefore the fluorescence depth will increase regularly.
Mild-activated oxidase-mimicking exercise of DTT@BSA-AuNCs. (a) Fluorescence spectra of the three samples containing DTT@BSA-AuNCs + TH with out mild irradiation, TH solely, and DTT@BSA-AuNCs + TH below mild irradiation. DTT@BSA-AuNCs + TH below mild irradiation displayed sturdy fluorescence emission peak at 440 nm. Nevertheless, DTT@BSA-AuNCs + TH with out mild irradiation and TH solely with mild irradiation, didn’t present any fluorescence (b) Photocontrollable oxidase-mimicking exercise of DTT@BSA-AuNCs demonstrated by the staircase-like conduct when the sunshine supply is turned on and off, as indicated by the blue and black arrows, respectively. (c) Oxidase-like photocatalytic actions of BSA-AuNCs synthesized by standard (BSA-AuNCs, dotted curves) and DTT-assisted (DTT@BSA-AuNCs, daring curves) strategies below totally different irradiation occasions. Coloration codes point out irradiation occasions: pink for 15 min, orange for 30 min, and violet for 60 min. (d) Dedication of accelerating fluorescence spectra with rising TH focus (as much as 1 mM) represented by lighter to darker shades of purple below a set BSA-AuNCs focus throughout 10 min of sunshine irradiation
To verify whether or not the inclusion of DTT might improve the oxidase-mimicking photocatalytic efficiency of BSA-AuNCs, we synthesized BSA-AuNCs within the presence of DTT (with a one-hour response at 50 °C) and within the absence of DTT (with a 12-hour response at 37 °C). Then, we incubated the ready DTT@BSA-AuNCs and BSA-AuNCs in TH and subjected them to prolonged mild irradiation for 15, 30, and 60 min. As displayed in Fig. 3c, we initially noticed related oxidase mimicking photocatalytic talents in each variants when the irradiation time was restricted to fifteen min. Nevertheless, apparently, because the irradiation time elevated to 30 and 60 min, a outstanding enhancement in photocatalytic efficiency was evident within the DTT@BSA-AuNCs. In distinction, conventionally synthesized BSA-AuNCs exhibited minimal enchancment in photocatalytic conduct, even with prolonged irradiation. This discovering confirmed the superior oxidase-mimicking photocatalytic effectivity of the DTT@BSA-AuNCs synthesized by way of the DTT-assisted methodology, which was probably attributed to elevated nanocluster formation. Due to this fact, DTT@BSA-AuNCs have been chosen for the current research. Current research on DTT-modified AuNCs for biosensing have demonstrated how DTT enhances the binding affinity for particular goal molecules reminiscent of mercury ions [51] and 6-mercaptopurine [52] by way of floor modification. This binding alters the fluorescence of the AuNCs, enabling detection. In distinction, our analysis targeted on the photocatalytic exercise of DTT-BSA-AuNCs. We explored how DTT influences the effectivity of light-driven reactions. Whereas earlier studies emphasize floor modification for goal binding, in our research, DTT acted as a lowering agent, resulting in the synthesis of extra AuNCs, leading to extra energetic websites for photocatalysis. Subsequent, the irradiation wavelength dependency of the reported photoresponsive oxidase-like exercise of DTT@BSA- AuNCs was investigated utilizing a number of LED wavelengths starting from ultraviolet to seen, to near-infrared mild (NIR), together with 365, 420, 470, 530, 570, 617, 735, and 940 nm. Determine S9 within the ESM exhibits that the UV-visible spectrum, particularly at wavelengths starting from 365 nm to 530 nm, was probably the most appropriate area for triggering the operate of the nanozyme, which decreased as the sunshine wavelength approached the NIR vary. Primarily based on these observations and contemplating the vary from 365 to 530 nm, the LED with a wavelength of 420 nm was chosen because the preliminary mild supply for the present research as a result of it falls inside this vary and is near the midpoint. Picture-responsive nanozymes have historically been activated by highly effective mild sources reminiscent of xenon lamps [53, 54]. This research, nevertheless, demonstrated that a regular LED emitting mild inside the UV-vis spectrum can successfully activate the reported nanozyme. Whereas Xenon lamps produce mild with a better photon flux and larger depth, our findings spotlight that LEDs, regardless of their decrease depth, stay a viable and extra sustainable different. Using LEDs presents benefits by way of diminished power consumption and upkeep necessities, enhancing the cost-effectiveness and environmental friendliness of the purposes.
To comprehensively perceive the mixed results of various concentrations of DTT@BSA-AuNCs, TH, and irradiation time, we carried out systematic experiments by which every parameter was adjusted. Initially, DTT@BSA-AuNCs have been used at 0.1 mM, TH concentrations starting from 0.1 to 1 mM, and irradiation occasions of 5, 10, 15, 20, and 25 min (Determine S10 within the ESM). Subsequently, DTT@BSA-AuNCs have been maintained at 1 mM, with related variations in TH concentrations and irradiation occasions (Determine S11 within the ESM). Within the subsequent set, the TH focus was fastened at 0.1 mM, the DTT@BSA-AuNCs focus was diverse from 0.1 to 1 mM, and the irradiation time was adjusted over a number of durations (Determine S12 within the ESM). Lastly, with TH fastened at 1 mM, the DTT@BSA-AuNCs concentrations have been diverse equally (Determine S13 within the ESM). The outcomes confirmed that the fluorescence depth of TC elevated proportionally with rising concentrations of DTT@BSA-AuNCs and TH, particularly with rising irradiation time. The abstract plots in Figures S10f, S11f, S12f, and S13f within the ESM spotlight these developments, demonstrating the tunability of the assay primarily based on these parameters. Furthermore, the noticed most fluorescence depth peak of TC, spanning from 425 nm to 445 nm, aligns with the anticipated attribute peak of TC, as reported by many different research [55,56,57]. Therefore, this in depth exploration confirmed the numerous skill of DTT@BSA-AuNCs to reinforce photoresponsiveness by rising the focus of DTT@BSA-AuNCs, TH, or irradiation time, thereby demonstrating the effectivity of the nanozyme. For additional research, the concentrations of DTT@BSA-AuNCs and TH have been maintained at 0.5 mM and 1 mM, respectively (until in any other case specified), with an irradiation time of 10 min. This alternative was made to cut back the consumption of the nanozyme and improve the cost-effectiveness of the purposes.
As displayed in Fig. 3d, upon rising the focus of TH whereas conserving the focus of DTT@BSA-AuNCs fastened, the fluorescence depth of TC regularly elevated till TH reached a focus of 1 mM throughout 10 min of irradiation. The rise within the fluorescence depth of TC demonstrated a linear relationship with an R2 worth of 0.99 (Determine S14a within the ESM), validates the effectiveness of our assay. This important linear correlation confirms that our fluorescence-based measurement precisely displays the focus of TC derived from TH, thereby establishing the assay’s reliability and robustness for exact quantitative evaluation. Nevertheless, when the focus of TH was additional elevated past 1 mM, a gradual lower in fluorescence depth was noticed (Determine S14b within the ESM). This intriguing phenomenon was attributed to ‘substrate inhibition,’ whereby at excessive substrate concentrations, the extreme substrate could nonspecifically bind to an alternate web site of the nanozyme, producing antagonistic results [58, 59].
To discover the flexibility of the photoregulated oxidase mimetic exercise of DTT@BSA-AuNCs, two extensively used chromogenic enzymatic substrates, 3,3,5,5-tetramethylbenzidine (TMB) and a couple of,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), have been examined. Determine S15a and b within the ESM exhibits that mild irradiation for 1 h might oxidize these enzymatic substrates into their respective oxidized varieties as noticed from their attribute absorbance peaks at 650 nm (TMBox) and 420 nm (ABTSox) within the UV-vis spectra. These outcomes demonstrated the versatile photoresponsive oxidase-like properties of DTT@BSA-AuNCs, with TH being probably the most delicate substrate as a result of it requires a shorter period of sunshine irradiation to drive its photocatalytic exercise than TMB and ABTS.
Catalytic mechanism of oxidase-mimicking DTT@BSA-AuNCs photocatalysis
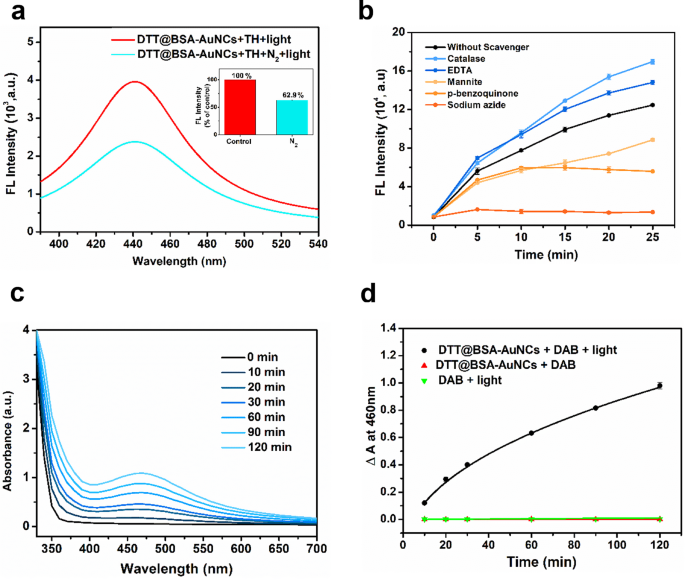
To quantify the oxidase-mimicking exercise of DTT@BSA-AuNCs, steady-state kinetic evaluation of the oxidation of TH was carried out. The standard kinetic parameters, such because the Michaelis-Menten fixed (Okm) and the utmost preliminary velocity (νmax), have been obtained by becoming the info to the Michaelis-Menten equation, and typical double-reciprocal Lineweaver-Burk plots have been constructed (Determine S16a and b within the ESM). Our research revealed a decrease Okm worth of 0.159 mM for the TH substrate than for the DTT@BSA-AuNCs, indicating the excessive affinity of the nanozyme for the substrate. Moreover, we noticed a considerably excessive νmax worth of three.79 × 10− 3 M/s, indicating the excessive catalytic effectivity of DTT@BSA-AuNCs. Comparisons of the Okm and νmax values obtained in our research with these reported in prior research are offered within the Desk S1 within the ESM. The decrease Okm and better νmax values obtained in our research point out the superior efficiency of our developed nanozyme system. To grasp the significance of dissolved oxygen within the photocatalytic exercise of DTT@BSA-AuNCs, we purged the response combination with nitrogen fuel to attenuate dissolved oxygen ranges, adopted by irradiation. As illustrated in Fig. 4a, a considerable lower (37.1%) in photocatalytic exercise was noticed upon oxygen depletion, suggesting that dissolved oxygen performs a significant function within the photocatalytic exercise of DTT@BSA-AuNCs. Nevertheless, the potential of residual dissolved oxygen within the purged combination can’t be completely dominated out.
Catalytic mechanism of DTT@BSA-AuNCs-TH system. (a) Fluorescence spectra exhibiting photocatalytic exercise of DTT@BSA-AuNCs within the presence of dissolved O2 (from air) and N2 (from N2 purging). Inset image exhibits the fluorescence depth at 440 nm expressed as % of management the place the management situation represents the fluorescence depth measured in dissolved O2 (from air), and the experimental group represents the fluorescence depth measured in presence of N2 (from N2 purging). (b) Impact of various ROS scavengers (catalase for H2O2, EDTA for h+, mannite for •OH, p-benzoquinone for O2•−, sodium azide for 1O2) on the photocatalytic oxidation on TH by the DTT@BSA-AuNCs below mild irradiation. (c). Absorbance spectra of DAB (1O2 probe) and DTT@BSA-AuNCs, with rising irradiation occasions from 10 min to 120 min represented by darker to lighter shades of blue. DAB with DTT@BSA-AuNCs exhibits elevated absorbance round 460 nm with extended irradiation. (d) Change in absorbance at 460 nm for DTT@BSA-AuNCs + DAB with and with out mild irradiation, and for DAB in presence of sunshine. Solely DTT@BSA-AuNCs + DAB below mild irradiation confirmed elevated absorbance at 460 nm. No change in absorbance at 460 nm was noticed for DTT@BSA-AuNCs + DAB with out mild and solely DAB in presence of sunshine. Error bars characterize normal deviation of three unbiased measurements
For the oxidase-like exercise of DTT@BSA-AuNCs, numerous reactive species might be concerned as reactive intermediates, reminiscent of hydroxyl radicals (•OH), singlet oxygen (1O2), hydrogen peroxide (H2O2), superoxide anions (O2•−), and photogenerated holes (h+). To establish the important thing reactive species accountable for TH oxidation, we employed selective quenchers to eradicate the ROS individually. Mannitol and catalase have been used to scavenge •OH and H2O2, respectively. Sodium azide was used for 1O2, p-benzoquinone for O2•−, and EDTA for h+. Determine 4b exhibits that sodium azide successfully inhibited TH oxidation fully, indicating the dominant function of 1O2 within the course of. P-benzoquinone and mannitol additionally inhibited TH oxidation however to a lesser extent than sodium azide. Apparently, EDTA enhanced the catalytic exercise as a result of it acts as a sacrificial gap acceptor, lowering the recombination of photogenerated electrons and holes and thereby rising the general oxidase-like exercise [60]. Moreover, the presence of catalase elevated TH oxidation, probably because of its peroxidase-like exercise at low H2O2 concentrations, which oxidizes the substrate [61, 62]. Therefore, these outcomes point out that 1O2 performs the dominant function within the photocatalytic conduct of DTT@BSA-AuNCs adopted by •OH and O2•−. To additional confirm the era of 1O2 from DTT@BSA-AuNCs upon mild irradiation, DAB was used as a 1O2 probe. As proven in Fig. 4c and d, DAB exhibited a rise in absorbance at roughly 460 nm upon response with 1O2, which elevated with extended irradiation time. Conversely, within the absence of sunshine, the absorbance of DAB didn’t enhance, indicating that mild is required for the DTT@BSA-AuNCs to launch 1O2. Due to this fact, these findings collectively point out that the first driver of the photocatalytic exercise of DTT@BSA-AuNCs is singlet oxygen.
Stability of the photoresponsive oxidase-mimicking exercise of DTT@BSA-AuNCs
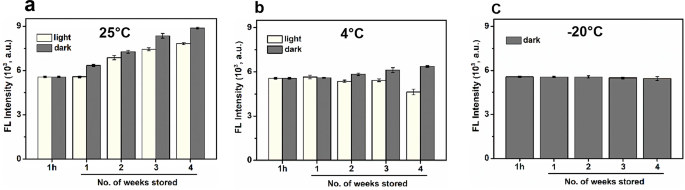
Pure enzymes sometimes lose stability and catalytic exercise over time, whereas nanozymes are anticipated to supply higher stability and long-term storage. To evaluate this, DTT@BSA-AuNCs have been saved at room temperature (25 °C), 4 °C, and − 20 °C for one month in each darkish and lighted environments. The photocatalytic exercise was monitored weekly (Fig. 5a-c). The outcomes point out that DTT@BSA-AuNCs remained steady and apparently, their catalytic exercise elevated over time. This enhance is probably going because of the temperature-dependent synthesis of DTT@BSA-AuNCs, which regularly enhanced the discount of AuNCs. The next inhabitants of DTT@BSA-AuNCs led to a larger oxidase-like catalytic exercise. This remark is additional supported by the truth that at a barely increased temperature (25 °C), the catalytic exercise was larger in comparison with 4 °C (Fig. 5a and b). At -20 °C, no change in catalytic exercise was noticed, indicating an entire arrest of the lowering property of BSA at this low temperature (see Fig. 5c).
Storage stability of DTT@BSA-AuNCs at totally different temperature situations. Stability of the photoresponsive oxidase-mimicking skill of DTT@BSA-AuNCs saved for one month at (a) 25 °C (room temperature), (b) 4 °C, and (c) -20 °C, in each darkish (represented by gray bars) and lighted (represented by faint yellow bars) environments. Fluorescence depth was recorded weekly. Error bars characterize normal deviation of three unbiased measurements
Detection of antioxidants
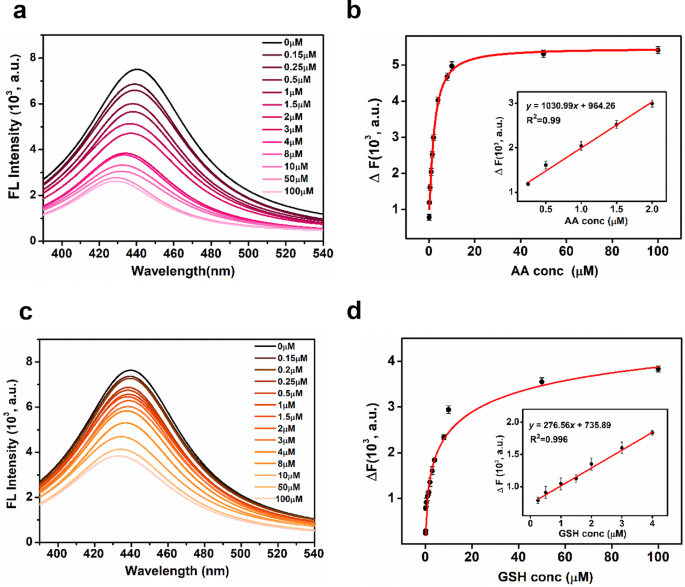
As famous in earlier mechanistic research mentioned on this article, the reason for TH oxidation by DTT@BSA-AuNCs within the presence of sunshine is strongly linked to the era of ROS by DTT@BSA-AuNCs. Antioxidants, acknowledged for his or her capability to neutralize free radicals or ROS, are anticipated to lower the oxidase mimicking functionality of DTT@BSA-AuNCs. To check the above speculation, we chosen ascorbic acid (AA), a typical and important antioxidant within the human physique, and glutathione (GSH), an vital organic thiol antioxidant discovered within the human physique, as mannequin antioxidants. As displayed in Fig. 6a and c, the fluorescence depth of oxidized TC decreased with rising concentrations of AA and GSH, which helps the rationality of earlier dialogue and demonstrates the detection feasibility. The change in fluorescence depth (∆F) of TC, measured because the distinction between its fluorescence within the absence (F0) and presence (F) of the antioxidants, AA and GSH, persistently elevated and remained steady when the AA and GSH concentrations have been above 10 µM (Fig. 6b and d). The ∆F reveals a linear relationship with AA focus within the vary of 0.25-2 µM, with a powerful correlation (R2 = 0.99) and a calibration curve described by the equation (:y=1030.99x+964.26) (discuss with the inset of Fig. 6b). Equally, for GSH within the vary of 0.25-4 µM, ∆F exhibits a powerful correlation (R2 = 0.996) with a calibration curve given by (:y=276.56x+735.89:) (discuss with the inset of Fig. 6d). The reproducibility of the assay is demonstrated by a mean coefficient of variation (CVav %) of three.5% for AA and 6.4% for GSH, indicating constant efficiency throughout a number of measurements.
Detection of AA and GSH. Fluorescence spectra of irradiated options of DTT@BSA-AuNCs and TH in PBS (pH 7.4) at totally different concentrations (0 − 100 µM) of (a) AA or (c) GSH present a lower in fluorescence depth with rising concentrations of the respective antioxidants. The connection between the change in fluorescence depth; ΔF and (b) AA focus or (d) GSH focus persistently elevated as much as 10 µM. The insets of (b) and (d) characterize a linear relationship with AA focus inside the vary of 0.25-2 µM, with a powerful correlation (R2 = 0.99), and with GSH focus inside the vary of 0.25-4 µM, with a powerful correlation (R2 = 0.996), respectively. Error bars exhibit the usual deviation of greater than three unbiased measurements
The bounds of detection (LOD) and limits of quantification (LOQ) for AA have been 0.08 µM and 0.26 µM, respectively whereas the LOD and LOQ for GSH have been 0.32 µM, and 0.98 µM, respectively. Because the lowest AA focus that may be quantitatively detected is 0.26 µM, and the focus of AuNCs within the assay is 500 µM, the Antioxidant-to-AuNC ratio is 0.00052:1. Which means 1 µM of AuNCs is succesful to detect 0.00052 µM of AA. Equally, the bottom GSH focus that may be quantitatively detected is 0.98 µM, giving an Antioxidant-to-AuNC ratio of 0.00196:1 which signifies 1 µM of AuNCs can detect 0.00196 µM of GSH. The Antioxidant-to-AuNC ratio for AA is considerably decrease than that of GSH, indicating increased sensitivity in direction of AA. That is additionally according to the obtained steeper slope of the calibration curve and decrease LOD for AA as in comparison with GSH. This distinction is attributed to the totally different antioxidant mechanisms of AA and GSH. AA, a major antioxidant and radical chain-breaker, quickly scavenges ROS, leading to larger fluorescence quenching. Whereas, GSH, being a secondary antioxidant, operates at a slower fee, resulting in a much less pronounced discount in fluorescence. This variation in response dynamics explains the noticed variations in sensing ranges between AA and GSH [63, 64].
Evaluating the efficiency of AA and GSH detection with beforehand reported outcomes, regarding LOD, detection time, and the medium of detection, as summarized in Desk S2 and Desk S3 within the ESM respectively, our findings reveal notable achievements. Notably, our ultralow LOD for AA outperforms all beforehand reported works, aside from one with a LOD of 0.01 µM (cited in Desk S2 within the ESM). Moreover, our LOD for GSH is superior to a number of reported values. In contrast to some standard assays, such because the FRAP assay which can’t detect thiol-containing antioxidants reminiscent of GSH because of its detection mechanism, our assay successfully detects GSH [4]. It’s because assays reminiscent of FRAP depend on a single electron switch (SET) response, whereas most physiologically related antioxidants, together with GSH, neutralize free radicals within the physique by way of hydrogen atom switch (HAT). Our assay overcomes this limitation, enabling GSH detection. Furthermore, ascorbic acid can make the most of each HAT and SET mechanisms [65]. This implies that our assay has the potential to detect a wider vary of antioxidants with various purposeful mechanisms.
It’s noteworthy that whereas all different research predominantly employed an acidic pH vary (pH 3 ̴ 5) because the medium of detection, our method utilized a impartial pH (pH 7.4), enhancing the sensible utility of the detection methodology. Using a light detection system at impartial pH and a brief detection time of 10 min contributed to the distinct attraction of our research demonstrating its promise for antioxidant detection.
Detection of antioxidants in human saliva
To additional lengthen the sensible utility of the detection technique, human saliva was chosen as a consultant organic pattern matrix. As depicted in Desk S4 within the ESM, the detection methodology proved efficient in detecting the mannequin antioxidants, AA and GSH in saliva. The desk signifies that the spiked AA and GSH restoration charges in saliva fall inside the ranges of 100.56-102.03% and 99.56-100.63% respectively. Given the presence of inherent antioxidants, reminiscent of AA and GSH, in human saliva [19], the noticed detection values barely exceeded the preliminary spiked quantity. The practically correct restoration outcomes obtained point out that the detection technique stays unaffected within the organic pattern matrix suggesting the robustness of the detection technique.
Selectivity of antioxidant detection
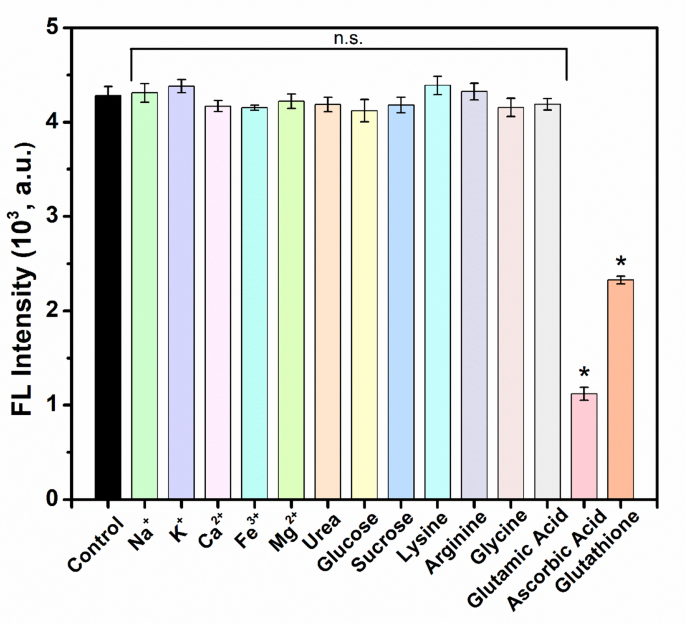
To be certified as an acceptable sensor materials, a nanozyme should not solely exhibit decrease sensitivity but in addition exhibit excessive selectivity. To judge the selectivity of the detection system for antioxidants, numerous related electrolytes generally present in human saliva reminiscent of sodium, potassium, calcium, iron, magnesium and customary biomolecules together with urea, glucose, sucrose and a few amino acids, reminiscent of lysine, arginine, glycine and glutamic acid have been chosen. The concentrations of the examined interferences have been stored the identical as these of the antioxidants studied, AA and GSH i.e., 100 µM making a standardized testing surroundings that allowed for a good comparability. As proven in Fig. 7, the fluorescence responses of the DTT@BSA-AuNCs-TH system to all of the interferents have been virtually unchanged in comparison with these of the management (clean answer). Nevertheless, on the identical focus of antioxidants, AA and GSH exhibited a major lower in fluorescence. Due to this fact, this detection technique reveals excessive selectivity for antioxidant detection.
Selectivity of the detection system in direction of the presence of antioxidants over numerous related interferences. Fluorescence spectra of irradiated options of DTT@BSA-AuNCs and TH in PBS (pH 7.4) with 100 µM of AA, GSH, or interferents reveal a lower in fluorescence depth solely within the presence of AA or GSH, however not within the presence of interferents. Every error bar exhibits the usual deviation of three unbiased measurements. *p < 0.01 in contrast with the management group; n.s. represents ‘not considerably totally different from the management group’
Detection of mobile antioxidants inside most cancers cells
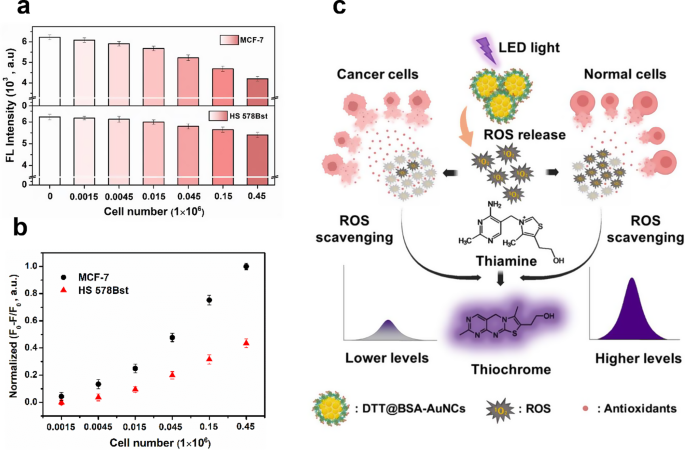
GSH is probably the most important endogenous antioxidant in cells. Elevated ranges of GSH are carefully related to most cancers growth, because it serves as a survival technique to counteract elevated oxidative stress in most cancers cells [38, 39, 66]. The rise in GSH ranges can also be linked to tumor promoters reminiscent of Nuclear issue erythroid 2-related issue 2 (NRF2) that activate the GSH synthesis [67]. This research employed DTT@BSA-AuNCs to evaluate the presence of antioxidants inside most cancers cells. MCF-7 cells, a mannequin breast most cancers cell line, have been in contrast with HS 578Bst cells, regular breast cell line. As proven in Fig. 8a, gradual lower within the attribute fluorescence depth of TC was noticed with rising numbers of each MCF-7 and HS 578Bst cells (1.5 × 103 to 4.5 × 105 cells/mL). This lower was because of the inhibition of the oxidase-like exercise of DTT@BSA-AuNCs by mobile antioxidants. Whereas most intracellular AA is understood to be depleted throughout cell tradition, the noticed lower aligns with the recognized skill of different mobile antioxidants, together with GSH, to quench the fluorescence of TC. Notably, MCF-7 cells exhibited roughly twice the inhibition impact in comparison with HS 578Bst cells (Fig. 8b). That is probably because of the presence of two-fold increased ranges of GSH in breast most cancers cells as in comparison with regular cells, reported in earlier research [36, 68]. A schematic illustration of detecting antioxidants in most cancers cells and regular cells is elucidated in Fig. 8c, displaying extra mobile antioxidants in most cancers cells successfully scavenges ROS, lowering thiamine to thiochrome conversion and leading to decrease fluorescence. Conversely, regular cells with much less mobile antioxidants results in much less ROS scavenging (rather more ROS buildup), rising thiamine to thiochrome conversion leading to increased fluorescence.
Detection of mobile antioxidants by DTT@BSA-AuNCs. (a) Lower in fluorescence depth of irradiated options of DTT@BSA-AuNCs and TH with rising concentrations of MCF-7 breast most cancers cells (higher panel) and HS 578Bst regular breast cells (decrease panel), with cell numbers starting from 1.5 × 103 to 4.5 × 105 cells/mL. (b) Plot of normalized (F0 − F)/F0 versus the cell quantity starting from 1.5 × 103 to 4.5 × 105 cells/mL; the place F0 represents fluorescence depth at 440 nm within the absence of cells and F represents fluorescence depth at 440 nm within the presence of cells. (c) Schematic illustration of the detection of antioxidants in most cancers cells (left aspect) versus regular cells (proper aspect). In most cancers cells, increased ranges of mobile antioxidants successfully scavenge ROS, which reduces thiamine to thiochrome conversion and ends in decrease fluorescence. In distinction, regular cells, with fewer mobile antioxidants, exhibit diminished ROS scavenging, resulting in elevated ROS buildup, increased thiamine to thiochrome conversion, and thus, increased fluorescence. Every error bar exhibits the usual deviation of three unbiased measurements
Due to this fact, this end result demonstrated the potential of DTT@BSA-AuNCs for detecting mobile antioxidant ranges, enabling various organic purposes.