Because the title suggests, most digital units at this time work by means of the motion of electrons. However supplies that may effectively conduct protons — the nucleus of the hydrogen atom — may very well be key to various necessary applied sciences for combating international local weather change.
Most proton-conducting inorganic supplies out there now require undesirably excessive temperatures to realize sufficiently excessive conductivity. Nonetheless, lower-temperature options may allow a wide range of applied sciences, resembling extra environment friendly and sturdy gas cells to supply clear electrical energy from hydrogen, electrolyzers to make clear fuels resembling hydrogen for transportation, solid-state proton batteries, and even new sorts of computing units primarily based on iono-electronic results.
With the intention to advance the event of proton conductors, MIT engineers have recognized sure traits of supplies that give rise to quick proton conduction. Utilizing these traits quantitatively, the workforce recognized a half-dozen new candidates that present promise as quick proton conductors. Simulations recommend these candidates will carry out much better than current supplies, though they nonetheless must be conformed experimentally. Along with uncovering potential new supplies, the analysis additionally offers a deeper understanding on the atomic stage of how such supplies work.
The brand new findings are described within the journal Vitality and Environmental Sciences, in a paper by MIT professors Bilge Yildiz and Ju Li, postdocs Pjotrs Zguns and Konstantin Klyukin, and their collaborator Sossina Haile and her college students from Northwestern College. Yildiz is the Breene M. Kerr Professor within the departments of Nuclear Science and Engineering, and Supplies Science and Engineering.
“Proton conductors are wanted in clear power conversion purposes resembling gas cells, the place we use hydrogen to supply carbon dioxide-free electrical energy,” Yildiz explains. “We wish to do that course of effectively, and subsequently we want supplies that may transport protons very quick by means of such units.”
Current strategies of manufacturing hydrogen, for instance steam methane reforming, emit an excessive amount of carbon dioxide. “One strategy to get rid of that’s to electrochemically produce hydrogen from water vapor, and that wants superb proton conductors,” Yildiz says. Manufacturing of different necessary industrial chemical compounds and potential fuels, resembling ammonia, will also be carried out by means of environment friendly electrochemical techniques that require good proton conductors.
However most inorganic supplies that conduct protons can solely function at temperatures of 200 to 600 levels Celsius (roughly 450 to 1,100 Fahrenheit), and even larger. Such temperatures require power to take care of and may trigger degradation of supplies. “Going to larger temperatures isn’t fascinating as a result of that makes the entire system more difficult, and the fabric sturdiness turns into a difficulty,” Yildiz says. “There is no such thing as a good inorganic proton conductor at room temperature.” At the moment, the one identified room-temperature proton conductor is a polymeric materials that’s not sensible for purposes in computing units as a result of it could possibly’t simply be scaled all the way down to the nanometer regime, she says.
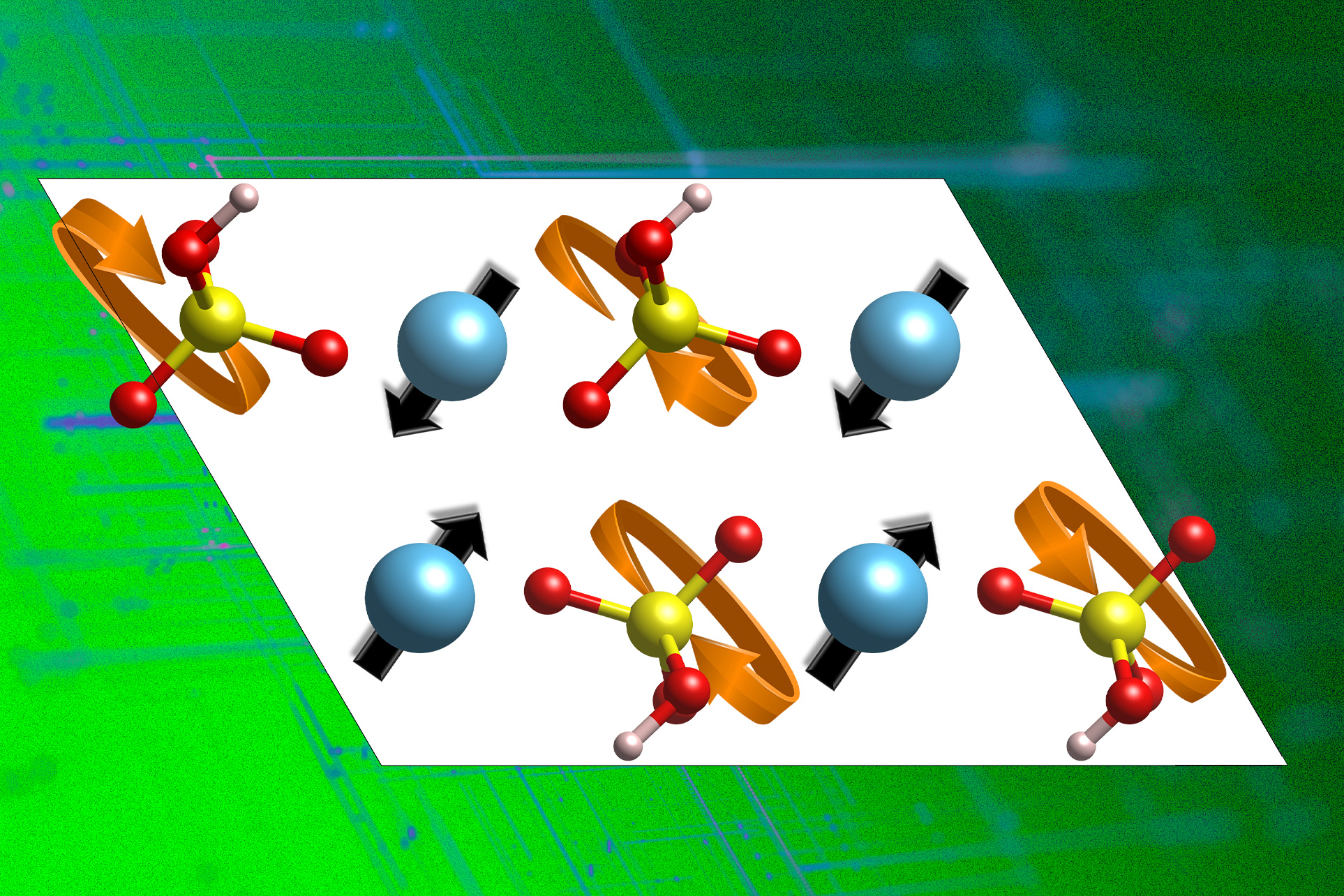
To sort out the issue, the workforce first wanted to develop a fundamental and quantitative understanding of precisely how proton conduction works, taking a category of inorganic proton conductors, known as stable acids. “One has to first perceive what governs proton conduction in these inorganic compounds,” she says. Whereas wanting on the supplies’ atomic configurations, the researchers recognized a pair of traits that instantly pertains to the supplies’ proton-carrying potential.
As Yildiz explains, proton conduction first includes a proton “hopping from a donor oxygen atom to an acceptor oxygen. After which the surroundings has to reorganize and take the accepted proton away, in order that it could possibly hop to a different neighboring acceptor, enabling long-range proton diffusion.” This course of occurs in lots of inorganic solids, she says. Determining how that final half works — how the atomic lattice will get reorganized to take the accepted proton away from the unique donor atom — was a key a part of this analysis, she says.
The researchers used pc simulations to check a category of supplies known as stable acids that turn into good proton conductors above 200 levels Celsius. This class of supplies has a substructure known as the polyanion group sublattice, and these teams must rotate and take the proton away from its unique website so it could possibly then switch to different websites. The researchers had been in a position to establish the phonons that contribute to the flexibleness of this sublattice, which is crucial for proton conduction. Then they used this data to comb by means of huge databases of theoretically and experimentally attainable compounds, in the hunt for higher proton conducting supplies.
In consequence, they discovered stable acid compounds which might be promising proton conductors and which have been developed and produced for a wide range of totally different purposes however by no means earlier than studied as proton conductors; these compounds turned out to have simply the suitable traits of lattice flexibility. The workforce then carried out pc simulations of how the precise supplies they recognized of their preliminary screening would carry out beneath related temperatures, to verify their suitability as proton conductors for gas cells or different makes use of. Positive sufficient, they discovered six promising supplies, with predicted proton conduction speeds sooner than the most effective current stable acid proton conductors.
“There are uncertainties in these simulations,” Yildiz cautions. “I don’t wish to say precisely how a lot larger the conductivity shall be, however these look very promising. Hopefully this motivates the experimental discipline to attempt to synthesize them in numerous types and make use of those compounds as proton conductors.”
Translating these theoretical findings into sensible units may take some years, she says. The seemingly first purposes could be for electrochemical cells to supply fuels and chemical feedstocks resembling hydrogen and ammonia, she says.
The work was supported by the U.S. Division of Vitality, the Wallenberg Basis, and the U.S. Nationwide Science Basis.