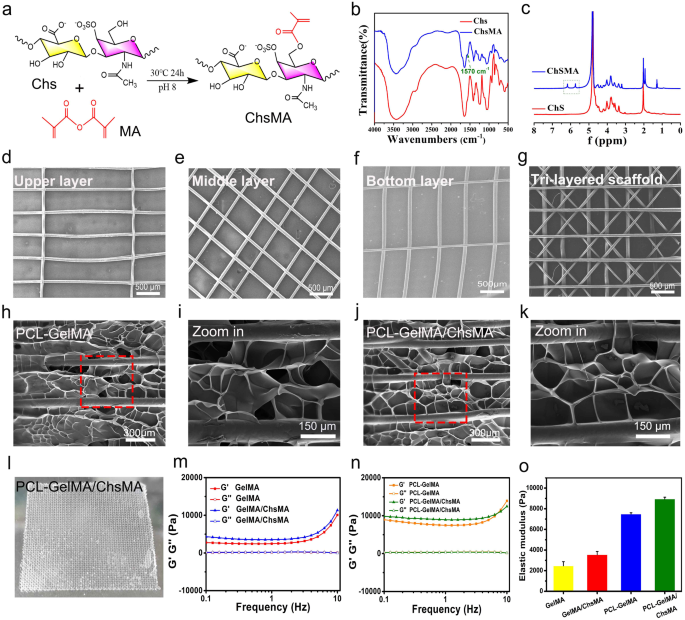
Biomimetic design and fabrication of melt-electrowriting scaffolds
Our examine goals to create an artificial leaflet with hydrogel-filled tri-layered PCL composite scaffold, which is meticulously designed to reflect the structural and mechanical attributes of pure coronary heart valves. Native coronary heart valve tissues exhibit intricate 3D buildings comprising a number of interconnected layers, and the structure of those layers performs a pivotal function in figuring out their mechanical traits and performance [39]. The essential mechanical properties of those tissues are anisotropic, which arises from the alignment of fibers throughout the extracellular matrix (ECM). The pure valve tissue primarily consists of collagen, with a small quantity of polysaccharide [34]. To simulate the composition of pure valves, we initiated the method by making ready GelMA and ChsMA molecules. These molecules had been synthesized by reacting gelatin and chondroitin sulfate (Chs) with methacrylic anhydride, as illustrated in Fig. 2a. Determine S1 illustrates the FTIR and 1H NMR spectra of gelatin and GelMA, revealing a novel peak at 1564 cm− 1 within the FTIR spectrum of GelMA and the emergence of two extra peaks at 5.6 and 5.4 ppm within the 1H NMR spectrum. These findings affirm the profitable grafting of methacrylic anhydride onto gelatin. ChsMA was additionally efficiently obtained, as confirmed by FTIR and 1H NMR spectra. The stretching band of the C = C bond from methacrylate at 1570 cm− 1 was detected in ChsMA (Fig. 2b). The presence of two distinct peaks at 5.6 and 6.1 ppm was ascribed to the 2 protons linked to the CH = CH2 bond of methacrylate (Fig. 2c). We then employed a Bio-3D printer outfitted with an electrostatic direct writing nozzle to manufacture numerous poly(ε-caprolactone) (PCL) MEW scaffolds. Initially, we designed and printed monophasic scaffolds, which included transverse-aligned fibers, diamond-shaped fibers, and vertically-aligned fibers architectures (Fig. 2d-f). Subsequently, we proceeded to print tri-layered anisotropic PCL MEW scaffolds in a single package deal (Fig. 2g). The imply fiber diameter throughout the constructs was decided to be 55 ± 1.5 μm. Notably, this dimension is smaller than the standard fiber diameters of the 3D-printed scaffolds manufactured utilizing standard soften extrusion strategies equivalent to fused deposition modeling and extrusion. Sometimes, these standard strategies end in fiber diameters exceeding 200 μm [40].
The MEW-PCL scaffolds had been built-in into hydrogel through molding, producing hybrid constructs that harness the strengths of every constituent. Exactly, these hybrid constructs derive benefits from the custom-made mechanical properties and biomimetic microarchitecture offered by the fiber section. The validation of those structural traits was completed by a morphological examination of SEM pictures (Fig. 2h-k). The GelMA and GelMA/ChsMA hydrogel, when launched into the PCL MEW scaffold, varieties a tightly certain dimensional pore construction. The precise look of this composite scaffold is depicted in Fig. 2l, revealing a troublesome, multilayered construction. A deeper understanding of the viscoelastic properties of those composite scaffolds was obtained by a frequency sweep evaluation overlaying a variety of frequencies (0.1–10 Hz). As illustrated in Fig. 2m-o, each GelMA and GelMA/ChsMA hydrogel exhibited comparable nonlinear rheological behaviors, with an elastic modulus of about 2000 Pa. It’s noteworthy that the storage modulus (G’) and loss modulus (G’’) noticeably elevated when MEW-PCL scaffold was embedded within the hydrogel.
Design and fabrication of MEW-enabled biomimetic anisotropic scaffolds for HVTE. (a) Diagram of modification response of chondroitin sulfate methacrylate. (b) FTIR spectra present the comparability earlier than and after the modification of chondroitin sulfate with methacrylate anhydride. (c) 1H NMR spectra of chondroitin sulfate and chondroitin sulfate methacrylamide. The SEM pictures show (d) transverse-aligned fibers on the highest layer, (e) diamond-shaped fibers on the center layer, and (f) vertically aligned fibers on the underside layer of the tri-layered anisotropic MEW-PCL scaffolds. (g) SEM pictures of tri-layered anisotropic MEW-PCL scaffolds. SEM pictures of the GelMA-hydrogel-filled MEW-PCL composite scaffold (h) and a neighborhood magnification picture (i). SEM pictures of the GelMA/ChsMA-hydrogel-filled MEW-PCL composite scaffold (j) and a neighborhood magnification picture (okay). (l) {A photograph} of GelMA/ChsMA-hydrogel-filled MEW-PCL composite scaffold. (m) G’ and G’’ of GelMA and GelMA/ChsMA scaffolds. (n) G’ and G’’ of PCL-GelMA and PCL-GelMA/ChsMA composite scaffolds. (o) The elastic modulus (G’) at 1 Hz of various scaffolds
Characterization of bioactive-hydrogel-embedded composite scaffold
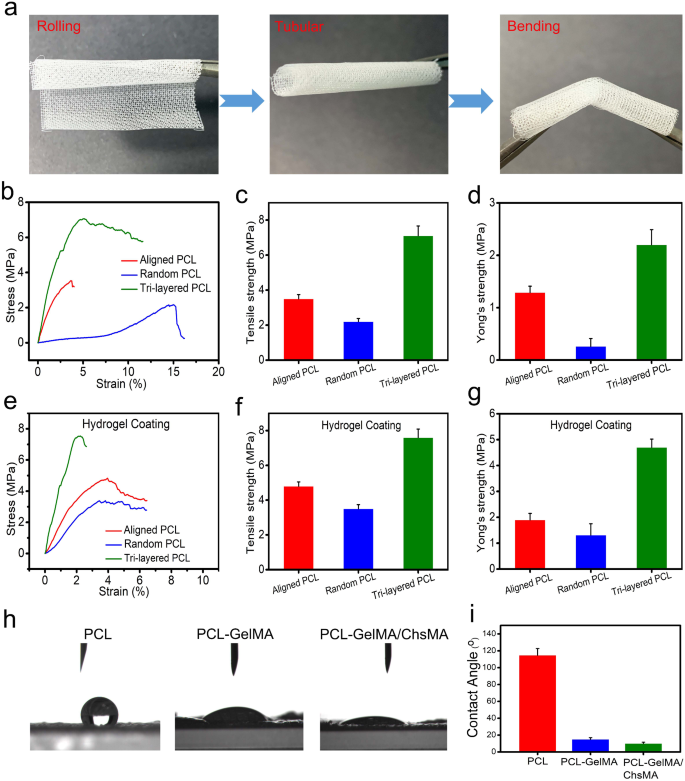
We carried out uniaxial tensile experiments to guage the affect of a exactly regulated anisotropic construction on the mechanical traits. Uniaxial testing has been also used in characterizing the mechanical conduct of native valvular tissue, [41] offering benchmark values important for the development of scaffolds in HVTE. Earlier than tensile testing, we do a crimp check on the scaffold to see how robust it’s. The result’s proven in Fig. 3a. MEW-PCL scaffold has good toughness, in order that it may be rolled right into a tube form and bent. To showcase the viability of using PCL-GelMA/ChsMA for the guts valves, a 3D-printed valve stent was manufactured. The valve stent, depicted in Determine S2a, possessed a diameter of 20 mm. The PCL-GelMA/ChsMA leaflet was positioned circumferentially and efficiently enveloped across the valve stent with the usage of cyanoacrylate. The leaflet placement resulted in an extra size of 5 mm above the valve put up, as measured. In Determine S2b-d, the mounted PCL-GelMA/ChsMA leaflet is noticed in open, semi-closed, and tightly closed states, demonstrating its potential for development as scaffolding for coronary heart valves.
Mechanical and hydrophilic properties of tri-layered anisotropic MEW-PCL scaffolds and the hydrogel-filled composite scaffolds. (a) Pictures of rolled, tubular, and bent tri-layered anisotropic MEW-PCL scaffolds. (b) Consultant stress-strain curves of every monophasic scaffold (aligned PCL fibers and random PCL fibers) and tri-layered anisotropic MEW-PCL scaffolds. (c) Younger’s modulus of every monophasic scaffold and tri-layered anisotropic MEW-PCL scaffolds. (d) Final tensile power of every monophasic scaffold and tri-layered anisotropic MEW-PCL scaffolds. (e) Consultant stress-strain curves of GelMA/ChsMA-hydrogel-filled tri-layered PCL composite scaffold. (f) Younger’s modulus of GelMA/ChsMA-hydrogel-filled tri-layered PCL composite scaffold. (g) Final tensile power of GelMA/ChsMA-hydrogel-filled tri-layered PCL composite scaffold. (h) Pictures of water droplet contacting totally different scaffolds. (i) The water contact angle of various scaffolds
Determine 3b reveals the tensile properties of the tri-layered MEW-PCL scaffold and its particular person layers. The tri-layered MEW-PCL scaffold displayed a markedly increased tensile power, registering at 7.1 ± 0.56MP, in comparison with the aligned PCL fibers (3.5 ± 0.24 MPa) and random PCL fibers (2.2 ± 0.18 MPa) (Fig. 3c). The Younger’s modulus of the MEW-PCL scaffold was (2.2 ± 0.29 MPa), which was notably increased for the aligned PCL fibers (1.29 ± 0.12 MPa) and random PCL fibers (0.26 ± 0.15 MPa) (Fig. 3d). The mechanical properties of GelMA/ChsMA-bioactive-hydrogel-embedded composite MEW-PCL scaffold have improved barely (Fig. 3e). Final tensile power of GelMA/ChsMA-hydrogel-embedded aligned PCL fibers and random PCL fibers was 4.8 ± 0.25 MPa and three.5 ± 0.23 MPa, respectively, whereas final tensile power of GelMA/ChsMA-hydrogel-embedded tri-layered MEW-PCL scaffold was 7.6 ± 0.49 MPa (Fig. 3f). The Younger’s modulus of the MEW-PCL scaffold additionally elevated with the embedment of the hydrogel. The Younger’s modulus of GelMA/ChsMA hydrogel-embedded aligned PCL fibers and random PCL fibers was 1.9 ± 0.24 MPa and 1.3 ± 0.42 MPa, whereas GelMA/ChsMA hydrogel-embedded tri-layered MEW-PCL scaffold was 4.7 ± 0.32 MPa (Fig. 3g). We additional evaluated the hydrophilicity of specimens by the water contact angle (Fig. 3h). The contact angles of MEW-PCL scaffold, PCL-GelMA scaffold, and PCL-GelMA/ChsMA had been 114.7°, 15.3°, and 10.6°, respectively, which indicated that hydrogels can considerably enhance the hydrophilicity of the tri-layered MEW-PCL scaffold.
In vitro cytotoxicity and endothelial cell development and proliferation
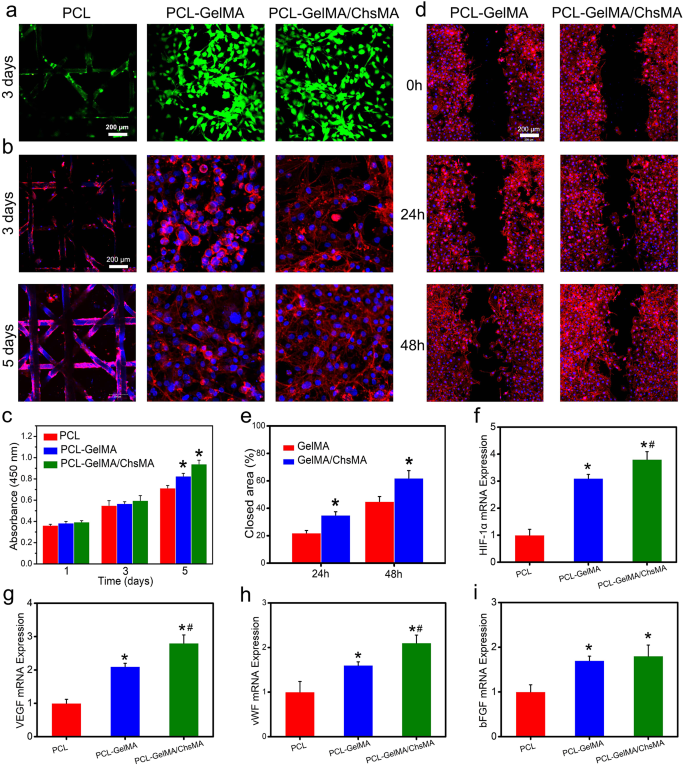
The combination of endothelial cells in bioprosthetic coronary heart valves holds important significance for anti-coagulant and anti-calcification functionalities [42]. To evaluate the potential for endothelialization, we utilized the HUVEC viability assay to evaluate the specimens [43]. Stay and useless staining of HUVECs grown on the floor of scaffolds reveals a major presence of viable cells on each the MEW-PCL scaffold and hydrogel-filled PCL scaffolds. This statement suggests the distinctive biocompatibility of each the MEW-PCL scaffold and the hydrogel-filled PCL scaffolds, as illustrated in Fig. 4a. Observations of HUVEC morphologies on the scaffolds had been carried out by actin staining. Determine 4b signifies that HUVECs exhibited glorious spreading and adhesion on the hydrogel-filled PCL scaffolds. Conversely, the MEW-PCL scaffold displayed bigger apertures, proving difficult to assist cell adhesion and development. Moreover, HUVECs assumed their typical elongated form on the hydrogel-filled PCL scaffolds. Moreover, mobile viability of HUVECs on the specimens was evaluated utilizing the CCK-8 assay (Fig. 4c). HUVECs cultured on hydrogel-filled PCL scaffolds displayed steady proliferation over a 5-day incubation interval, exhibiting increased absorbance values in comparison with these on the MEW-PCL scaffold. These outcomes recommend that the hydrogel-filled PCL scaffolds enhanced the proliferation of HUVECs. Altogether, these findings spotlight the GelMA/ChsMA hydrogel’s functionality in offering a biocompatible microenvironment conducive to HUVEC development.
Organic behaviors of HUVECs on the HVTE scaffolds. (a) Stay and useless staining of HUVECs grown on the floor of MEW-PCL scaffold and hydrogel-filled MEW-PCL scaffold after incubation for 3 days. (b) F-actin and DAPI staining of HUVECs grown on the floor of scaffolds after 3 and 5 days of incubation. (c) Cell viability of HUVECs on the floor of scaffolds after 1, 3 and 5 days of incubation. (d) Consultant pictures of scratch wound therapeutic assay at 0, 24, and 48 h. (e) Share of wound closure decided by the microscopic pictures. (f-i) The mRNA expression degree of HIF-1α, VEGF, vWF and bFGF in HUVECs after culturing with totally different scaffolds for 7 days. The offered knowledge are expressed as means ± SD (n = 6). A significance degree of p < 0.05* denotes a major distinction in comparison with the PCL group, whereas p < 0.05# signifies a major distinction compared to the PCL-GelMA group
Subsequent, cell migration check and RT-qPCR evaluation had been used to investigate the endothelialization of HUVECs on hydrogel-embedded scaffolds. Mobile migration performs a vital function in wound therapeutic, significantly for HUVECs, because it accelerates the endothelialization course of [44]. In Fig. 4d, it’s evident that cell migration was notably enhanced within the PCL-GelMA/ChsMA group when in comparison with the PCL-GelMA group. On the 48-hour mark, the PCL-GelMA/ChsMA group exhibited a considerably improved closure impact. Quantitative evaluation additional indicated that the PCL-GelMA/ChsMA group demonstrated a superior closure impact in comparison with the PCL-GelMA group (Fig. 4e), which needs to be principally attributable to bioactive chondroitin sulfate. Endothelial development components are vital within the modulation of endothelial cell behaviors. Subsequently, we additional investigation the expression of endothelial development factor-related genes in HUVECs. After culturing HUVECs on hydrogel-embedded PCL scaffolds for 7 days, we evaluated the gene expression ranges of HIF-1α, VEGF, vWF, and bFGF. The gene expression evaluation of HUVECs cultivated on PCL-GelMA/ChsMA reveals an elevated degree of gene expression, particularly for HIF-1α, VEGF, vWF, and bFGF, as illustrated in Fig. 4f-i. These findings point out that the PCL-GelMA/ChsMA scaffold displays favorable biocompatibility, and helps the expansion and proliferation of endothelial cells. Consequently, these outcomes place the PCL-GelMA/ChsMA scaffold as a promising candidate for the applying of TEHVs.
In vitro biocompatibility and hemocompatibility
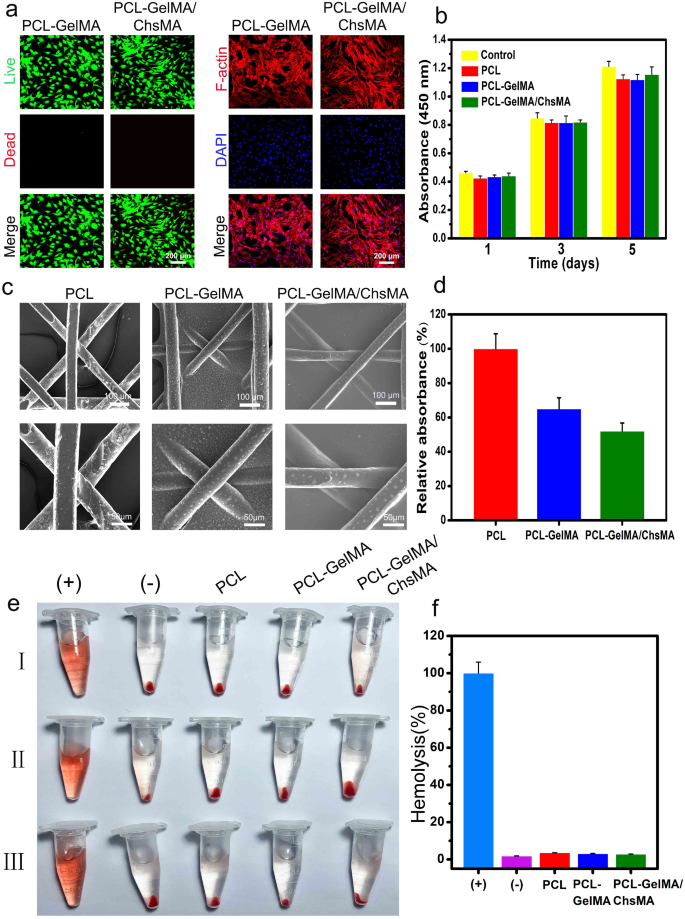
Hemocompatibility is a crucial evaluation parameter for supplies meant for blood contact [45, 46]. Earlier than delving into the investigation of the hemocompatibility, the biocompatibility of MEW-PCL scaffolds to VICs was examined. As depicted in Fig. 5a, stay/dead-stained pictures of VICs on PCL-GelMA and PCL-GelMA/ChsMA scaffolds reveal a major presence of residing cells on each scaffolds, indicating glorious biocompatibility for each PCL-GelMA and PCL-GelMA/ChsMA scaffolds. The morphologies of VICs on the scaffolds had been additional examined by actin staining. As depicted in Fig. 5a, the VICs exhibited good spreading and attachment on the scaffolds. Furthermore, the biocompatibility of the hydrogels was assessed utilizing the CCK-8 assay (Fig. 5b). VICs cultured on the scaffolds displayed steady proliferation over a 5-day incubation interval, with no important distinction noticed in comparison with the management group (tradition plate). This means that each the PCL scaffold and hydrogel-filled PCL scaffolds weren’t cytotoxic to VICs. These findings underscore that the PCL scaffold and hydrogel-filled PCL scaffolds present a biocompatible microenvironment conducive to the expansion of VICs.
Biocompatibility and hemocompatibility of the HVTE scaffolds. (a) Stay & useless staining and F-actin staining of VICs seeded on PCL-GelMA and PCL-GelMA/ChsMA scaffolds after a 5-day incubation interval. (b) Cell viability of VICs on the floor of scaffolds after incubation for 1, 3 and 5 days. (c) SEM pictures of PCL, PCL-GelMA and PCL-GelMA/ChsMA scaffolds after platelet adhesion assay. (d) Quantitative outcomes of platelets adsorbed on PCL, PCL-GelMA, and PCL-GelMA/ChsMA scaffolds, which was decided by the LDH launch assay. (e) Hemolysis outcomes of various scaffolds (“+” indicating the optimistic management group and “-” indicating the detrimental management group). (f) The hemolysis ratio of various scaffolds
To analyze the hemocompatibility of the PCL-GelMA and PCL-GelMA/ChsMA scaffolds, SEM was employed to look at platelet adhesion on these scaffolds. In Fig. 5c, quite a few platelets adhered to the floor of the PCL scaffold, whereas only some had been noticed on the surfaces of PCL-GelMA and PCL-GelMA/ChsMA scaffolds. The lactate dehydrogenase (LDH) launch assay additional demonstrated that the platelet adhesion on PCL-GelMA and PCL-GelMA/ChsMA scaffolds was considerably decrease than that on the PCL scaffold (Fig. 5d). Moreover, the hemolysis check outcomes proven in Fig. 5e indicated no obvious hemolysis phenomenon within the PCL, PCL-GelMA, and PCL-GelMA/ChsMA scaffold teams. The hemolysis ratios for PCL, PCL-GelMA, and PCL-GelMA/ChsMA scaffolds had been 3.6%, 3.1%, and a pair of.8%, respectively (Fig. 5f). These hemolysis charges had been properly under 5%, assembly the necessities of ISO 5840-3.2013 [47]. This underscores the passable hemocompatibility of the PCL, PCL-GelMA, and PCL-GelMA/ChsMA scaffolds.
In vitro organic exercise analysis of the HVTE scaffold
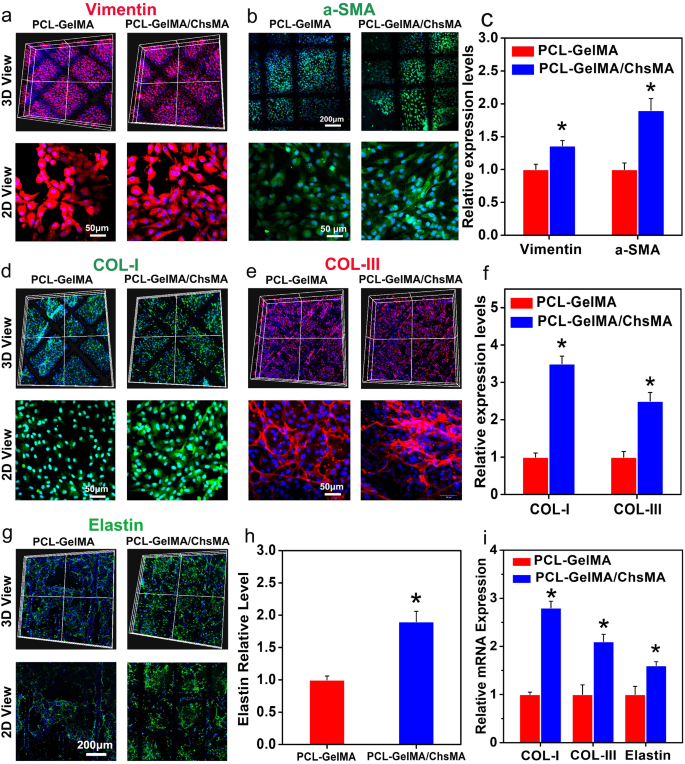
To guage the suitability of PCL-GelMA/ChsMA scaffolds for HVTE, GelMA/ChsMA hydrogels laden with major VICs had been included into MEW-PCL scaffold by molding. This technique produced hybrid constructs that leverage the custom-made mechanical traits and biomimetic microarchitecture supplied by the PCL fiber section, together with the heightened ECM manufacturing generally noticed in cell-laden hydrogels. Earlier studies have indicated that wholesome grownup VICs show a quiescent fibroblast-like phenotype, expressing vimentin and, to a lesser extent, αSMA (a myofibroblast marker) [48, 49]. Nonetheless, valves subjected to irregular hemodynamic circumstances have proven the presence of activated VICs. These activated VICs, recognized as myofibroblasts, provoke a proliferation course of and actively take part within the in depth transforming of the ECM. [50] As illustrated in Fig. 6a-c, the VICs encapsulated inside all hydrogel samples displayed the expression of each α-SMA and vimentin following a 14-day cultivation interval, signifying the transition of VICs from a fibroblastic to a myofibroblastic phenotype.
Immunofluorescence evaluation of the extracellular matrix transforming of the 3D-cultured VIC cells. (a) Vimentin expression offered by the VIC cells 3D-cultured inside PCL-GelMA and PCL-GelMA/ChsMA scaffolds. (b) a-SMA expression offered by the VIC cells 3D-cultured inside PCL-GelMA and PCL-GelMA/ChsMA scaffolds. (c) Quantitative evaluation of the immunostaining pictures in (a) and (b). (d) COL-I expression offered by the VIC cells 3D-cultured inside PCL-GelMA and PCL-GelMA/ChsMA scaffolds. (e) COL-II expression offered by the VIC cells 3D-cultured inside PCL-GelMA and PCL-GelMA/ChsMA scaffolds. (f) Quantitative evaluation of the immunostaining pictures of (d) and (e). (g) Elastin expression offered by the VIC cells 3D-cultured inside PCL-GelMA and PCL-GelMA/ChsMA scaffolds. (h) Quantitative evaluation of the immunostaining pictures in (g). (i) The mRNA expression degree of COL-I, COL-III and Elastin in VICs after culturing inside totally different scaffolds for 14 days. Information signify means ± SD (n = 6). p < 0.05* signifies a major distinction in contrast with the PCL-GelMA group
To additional assess the ECM deposition and transforming capabilities of VICs seeded within the hydrogel-embedded PCL scaffolds, organic assays had been carried out to quantify the contents of collagen I, collagen III, and elastin after 14 days of tradition. The incorporation of ChsMA facilitated elevated deposition of collagen and elastin. As depicted in Fig. 6d-f, VICs throughout the PCL-GelMA/ChsMA scaffold exhibited considerably increased ranges of collagen I and collagen III in comparison with these throughout the PCL-GelMA scaffold. Moreover, VICs within the PCL-GelMA/ChsMA scaffold additionally demonstrated considerably increased ranges of elastin in comparison with these within the PCL-GelMA scaffold (Fig. 6g, h). The gene expression ranges of collagen I, collagen III, and elastin had been evaluated after 14 days of VICs tradition in scaffolds. VICs cultured in PCL-GelMA/ChsMA demonstrated a heightened gene expression for collagen I, collagen III, and elastin, as illustrated in Fig. 6i. These findings substantiate our speculation that the synergistic mixture of bioactive GelMA/ChsMA hydrogel with anisotropic PCL MEW scaffolds has a optimistic influence on cell-scaffold interactions, significantly when it comes to ECM secretion and deposition.
The anticalcification means of engineered scaffolds is an assuredly vital property for HVTE scaffold. GelMA/ChsMA hydrogels laden with VICs had been included into MEW-PCL scaffold and cultured in a pro-osteogenic atmosphere utilizing osteogenic differentiation medium (ODM) for 14 days to discover how the scaffold elements affected the osteogenic differentiation of VICs. ODM helps VICs proliferation, with no important cytotoxicity noticed (Determine S3a). It was discovered that giant areas of Alizarin Purple staining (ARS) and quantitative evaluation had been noticed on each the PCL and PCL-GelMA scaffolds, decrease ARS was detected on the PCL-GelMA/ChsMA scaffold (Determine S3b, c). To additional validate the phenotypic change of VICs seeded within the three totally different HVTE scaffolds in ODM, IF staining was carried out to visualise the expression of Runx2 (a necessary transcription issue for osteogenic differentiation), (Determine S3d, e). The VICs cultured within the PCL-GelMA/ChsMA scaffold exhibited the bottom expression of Runx2. RT-qPCR evaluation was utilized to find out the expression of two gene markers, together with alkaline phosphatase (ALP) and Runx2. It was discovered that the VICs cultured within the PCL-GelMA/ChsMA scaffold considerably downregulated the expressions of ALP and Runx2 genes in comparison with the VICs cultured within the PCL and PCL-GelMA scaffolds (Determine S3f, g). These outcomes demonstrated that the introduction of ChsMA into the HVTE scaffolds successfully decreased VICs calcification.
In vivo immuno-inflammation and calcification evaluation of the scaffolds
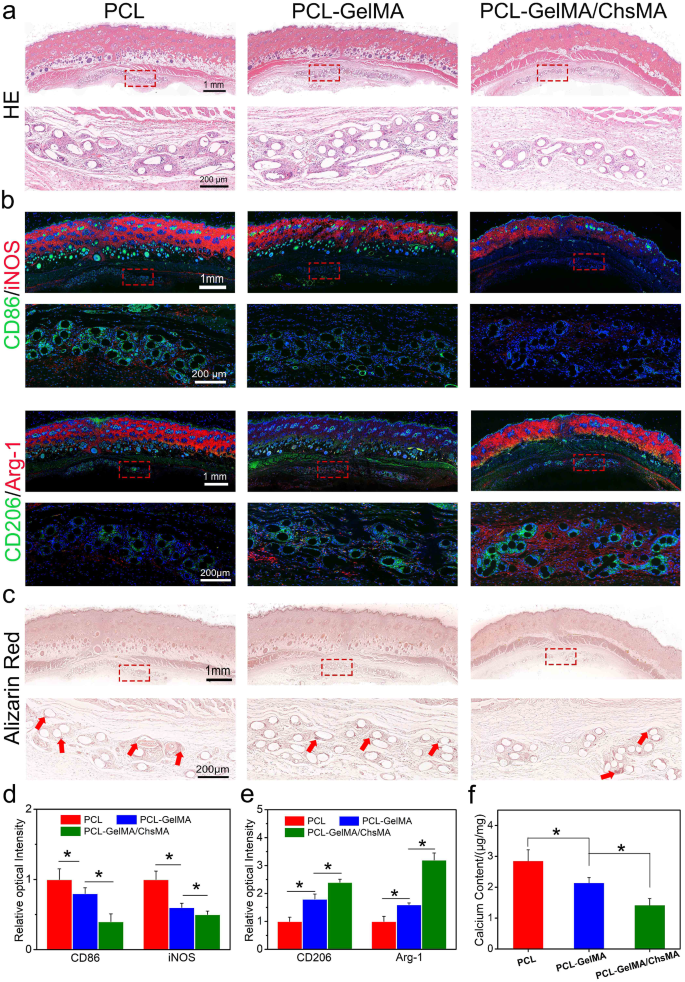
The rat subcutaneous implantation mannequin was employed to guage mobile infiltration, irritation, and calcification resistance in vivo. Determine 7a presents a sequence of hematoxylin and eosin (H&E) staining pictures depicting PCL, PCL-GelMA, and PCL-GelMA/ChsMA scaffolds. After 30 days of implantation, all three sorts of scaffolds displayed a dense tissue capsule surrounding the samples. The PCL fibers, being non-dyeable, resulted in a porous illustration of fiber cross-sections within the sections. H&E staining aided in distinguishing nuclei, showing as deep purple, and infiltrated connective tissue, exhibiting pink staining. Remarkably, Fig. 7a observations revealed the absence of a major inflammatory response or harm within the adjoining tissues, indicating that each one PCL scaffolds exhibited good in vivo biocompatibility.
Immune responses induced by PCL, PCL-GelMA and PCL-GelMA/ChsMA scaffolds after subcutaneous implantations for 4 weeks. (a) H&E staining of the scaffolds after 4-week implantation. (b) Consultant immunofluorescence staining pictures of the scaffolds after 4-week implantation (CD86/iNOS for M1 subtype macrophage, CD206/Arg-1 for M2 subtype macrophage). (c) Alizarin pink staining confirmed calcification of the scaffolds after 4-week implantation (pink arrows signify calcification). (d-e) Quantitative evaluation of the immunostaining pictures of CD86, iNOS, CD206 and Arg-1. (f) Calcium contents of PCL, PCL-GelMA and PCL-GelMA/ChsMA scaffolds after 4-week implantation, and the all samples had been measured by ICP. Information signify means ± SD (n = 6). p < 0.05* signifies a major distinction
Macrophages are adaptable cells able to transitioning into numerous states when uncovered to totally different stimuli. To characterize the polarization of macrophages after a 30-day implantation, triple-labeled immunofluorescence was carried out. M1-polarized macrophages had been recognized through co-staining of CD86 and iNOS, whereas M2-polarized macrophages had been labeled by CD206 and arginase-1 (Arg-1). Notably, fewer M1-polarized macrophages and an elevated presence of M2 macrophages had been noticed round PCL-GelMA/ChsMA scaffolds in comparison with the opposite teams (Fig. 7b-e). These findings steered a bent for macrophages round PCL-GelMA/ChsMA scaffolds to polarize in the direction of the M2 phenotype. The favorable anti-inflammatory efficiency of PCL-GelMA/ChsMA scaffolds may be attributed to the anti-inflammatory properties of chondroitin sulfate, a sulfur-containing polysaccharide recognized for its sure anti-inflammatory and immunomodulatory capabilities [51, 52]. Moreover, the wonderful biocompatibility of the hydrogel coating could contribute to the decreased chance of immune rejection.
Sections of the three samples exhibited indicators of calcification in each the supplies and their surrounding tissues (Fig. 7c). Following a 30-day implantation interval, alizarin pink staining revealed calcification deposits (dark-red parts) on the PCL scaffold, whereas no calcification was noticed on PCL-GelMA or PCL-GelMA/ChsMA scaffolds. The calcification additionally appeared to increase into the encompassing tissues, significantly on the interface between the tissue and the PCL scaffold. In the remainder of the encapsulated tissue, little to no calcification was current. To validate these histological findings, a calcium content material assay was carried out. ICP-OES was utilized for quantitative evaluation of calcification. After a 30-day implantation in SD rats, the calcium content material of PCL, PCL-GelMA, and PCL-GelMA/ChsMA scaffolds was measured at 2.8, 2.1, and 1.7 µg/mg, respectively (Fig. 7f). This consequence signifies that PCL-GelMA/ChsMA displays potential anti-calcification properties.
Total scaffold efficiency underneath hemodynamic circumstances
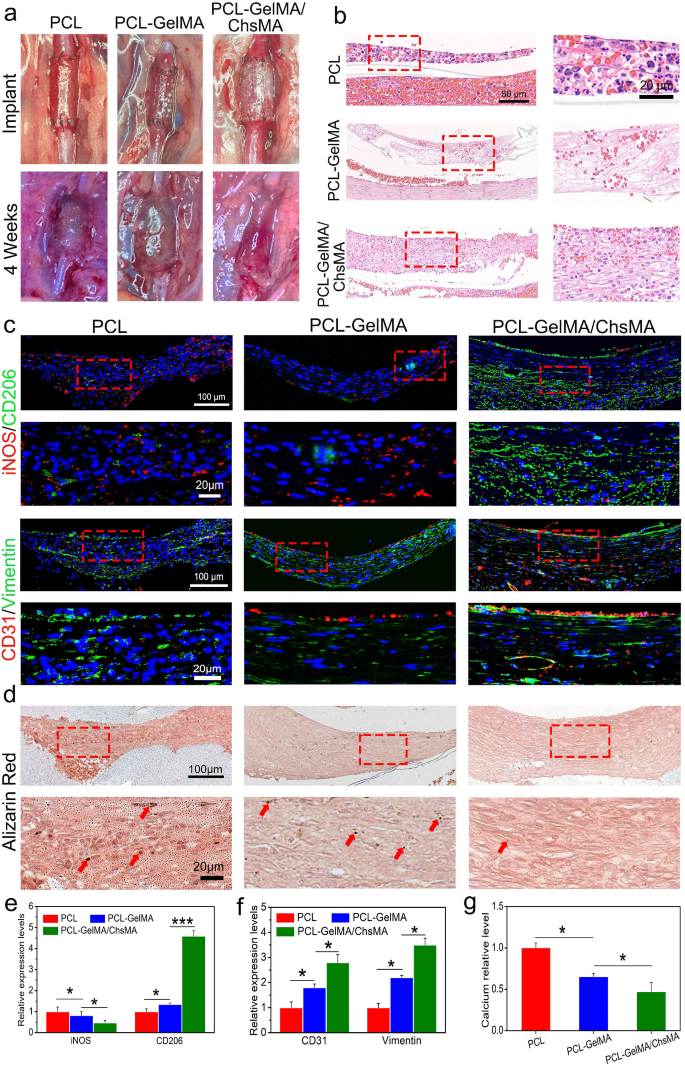
To guage scaffold efficiency in a hemodynamic atmosphere, we established a rat stomach aorta implantation mannequin. After 4 weeks of implantation, the PCL scaffold exhibited indicators of thrombogenesis (Fig. 8a) and underwent important dilation, resembling an aneurysm, whereas the PCL-GelMA and PCL-GelMA/ChsMA scaffolds maintained their tubular construction. Hematoxylin and eosin (H&E) staining pictures (Fig. 8b) revealed a layer of cell infiltration on the interior floor of the scaffold. Notably, the cells infiltrating the interior floor of the PCL scaffold exhibited the next presence of inflammatory cells, whereas the PCL-GelMA and PCL-GelMA/ChsMA scaffolds displayed decreased inflammatory cell infiltration. These findings point out that the PCL-GelMA and PCL-GelMA/ChsMA scaffolds exhibited favorable biocompatibility throughout the hemodynamic atmosphere.
Institution of the stomach aorta mannequin and total evaluation of the HVTE scaffolds after a 4-week implantation. (a) Photographic illustration of the scaffolds implanted within the rat stomach aorta. (b) H&E staining of the scaffolds after 4-week implantation. (c) Immunofluorescence pictures of iNOS and CD206 illustrating inflammatory infiltration, CD31 and vimentin displaying cellularization of the scaffolds. (d) Alizarin pink staining revealed the incidence of calcification within the scaffolds (pink arrows signify calcification). (e-g) Semi-quantitative evaluation of immunostaining pictures of iNOS, CD206, CD31, Vimentin and calcification. Information signify means ± SD (n = 6). p < 0.05*, p < 0.01*** signifies a major distinction
Put up-implantation irritation of the scaffolds was evaluated utilizing immunofluorescence staining for iNOS (indicative of M1 subtype macrophages) and CD206 (indicative of M2 subtype macrophages) (Fig. 8c, e). Total, all scaffolds exhibited a point of irritation, with PCL scaffolds demonstrating a extra pronounced inflammatory response in comparison with PCL-GelMA and PCL-GelMA/ChsMA scaffolds. Following a 4-week implantation interval, CD206 M2 macrophages, indicative of immunoregulation, dominated throughout the scaffolds, whereas the presence of iNOS M1 macrophages, related to immune stimulation, was minimal. This statement suggests a subsiding acute inflammatory response, with ongoing tissue transforming within the scaffolds at this juncture. In distinction to PCL and PCL-GelMA scaffolds, PCL-GelMA/ChsMA scaffolds displayed an earlier transition from the acute inflammatory section to tissue restore and transforming, suggesting their potential anti-inflammatory performance.
Endothelialization holds paramount significance for TEHV scaffolds. Its significance stems not solely from its very important function in integrating and regenerating TEHV to imitate a local valve but in addition from its various physiological features. These features embody the upkeep of blood homeostasis, the formation of an immune barrier, and the regulation of the phenotype of VICs [52]. Immunofluorescence staining for CD31 and vimentin was employed to visualise endothelialization and cellularization (CD31 for endothelial cells and vimentin for interstitial cells). As depicted in Fig. 8c and f, CD31 staining revealed the formation of a confluent CD31 + endothelial cell monolayer within the PCL-GelMA/ChsMA scaffold, albeit with some incomplete sections nonetheless current. The interior floor of the PCL-GelMA scaffold exhibited solely a sparse presence of endothelial cells, whereas no endothelialization was noticed within the PCL scaffold. Vimentin staining outcomes steered that each PCL-GelMA and PCL-GelMA/ChsMA scaffolds underwent cellularization, whereby endothelial cells fashioned a monolayer on the interior floor, and interstitial cells infiltrated into the principle physique of the scaffolds. This noticed sample resembled the mobile composition and construction of native valves.
Alizarin pink stain was employed to evaluate the calcification of those scaffolds underneath a hemodynamic atmosphere. After 4-week implantation, alizarin pink stained calcification deposits (brown parts) had been noticed within the PCL scaffold group, and some calcifications had been current within the PCL-GelMA scaffold group (Fig. 8d, g). In distinction, no seen brown parts had been detected on PCL-GelMA and PCL-GelMA/ChsMA, signifying the absence of great calcification after 4-week implantation. In consequence, we launched an revolutionary method by suturing the scaffold right into a tubular graft, which was subsequently implanted within the stomach aorta to partially emulate the hemodynamic atmosphere of a local valve. This mannequin demonstrates that the PCL-GelMA/ChsMA scaffold possesses immunomodulatory results, glorious hemocompatibility, and endothelialization, significantly when it comes to recruiting and capturing endothelial cells from peripheral blood sources, coupled with anti-calcification means.